The parasitic Nematode Group
Project leader:
Adrian Streit
Department:
IV - Integrative Evolutionary Biology
Director:
Ralf. J. Sommer
Office:
Maria Gölz
Max-Planck-Ring 9
D-72076 Tübingen
Germany
Phone: +49 7071 601 441
Fax: +49 7071 601 498
Group members:
- Veroni de Ree (doctoral student)
- Zhendong Du (visiting docotoral student)
- Dorothee Harbecke (technical assistent)
- Sandra Gyarteng (co-supervised doctoral student in the DAAD "Bi-nationally Supervised Doctoral Degrees" program; main institution Kwame Nkrumah University of Science and Technology at Kumasi, Ghana; main supervisor Prof. J. A. Larbi)
- Xiaoxiao Yin (doctoral student)
Alumni:
- Kittipat Aupalee (visiting PhD student)
- Anna Dyka (Bachelor student)
- Alex Dulovic (PhD student)
- Alexander Eberhardt (Diploma and PhD student)
- Li Guo (PhD student)
- Julia Hildebrandt (Diploma and PhD student)
- Anja Holz (Master and PhD student)
- Tegegn Jaleta (PhD student)
- Stephan Knierer (PhD student)
- Arpita Kulkarni (PhD student)
- Ryuji Minasaki (PhD student)
- Linda Nemetschke (PhD student)
- Veysi Piskobulu (PhD student, joint with Ralf Sommer)
- Viktoria Wegewitz (tech. ass. and PhD student)
- Erhan Yalcindag (visiting PhD student)
- Olga Zhukova (PhD student)
- Siyu Zhou (PhD student)
Projects
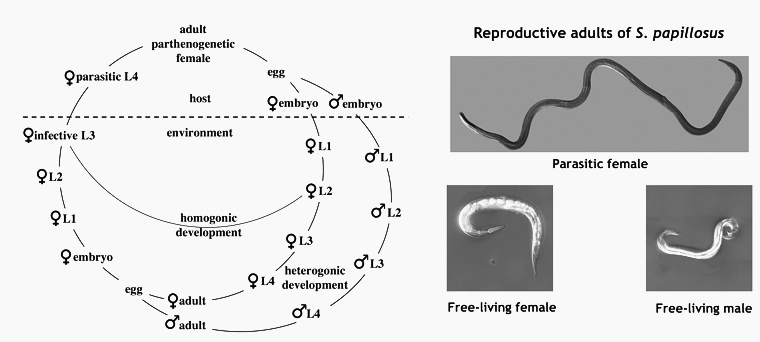
Strongyloides spp. a parasitic nematode with a facultative free-living generation
The nematode genus Strongyloides consists of parasites that live as parthenogenetic females in the small intestines of their vertebrate hosts. In addition to producing parasitic offspring, Strongyloides spp. can also form a facultative free-living generation with males and females. A generalized life cycle of Strongyloides sp. is shown in Figure 1. For a general introduction into the biology of Strongyloides sp. by Mark E. Viney and James B. Lok click here. We started our Strongyloides work in the laby with S. papillosus, a common parasite of sheep and goats, which can be raised in rabbits and S. ratti, a parasite of rats. More recently, in order to bring our laboratory and field projects closer together, we added the human parasite S. stercoralis, which we maintan in Mongolian Gerbils as laboratory hosts. For comparison, we also maintain free living cultures of Parastrongyloides trichosuri, a closely related facultative parasite of Australian possums and the non parasitic close relative Rhabditophanes diutinus (previously known as Rhabditophanes. sp. KR3021, Dulovic et al. 2020).
Tool and resource development
Since Strongyloides spp. are not yet established as research models like, for example, the model nematode Caenorhabditis elegans, part of our efforts are dedicated to the developmetn of tools and resources for Strongyloides research. Classical genetic approaches are rarely used with metazoan endo-parasites, largely because the adult stages are usually hidden within hosts, making controlled crosses difficult. The existence of a free-living generation in Strongyloides spp. offers a remarkable opportunity for the experimental manipulation of a parasite. We would like to explore this opportunity and conduct genetic screens in Strongyloides spp. We established a genetic linkage map for S. ratti (in collaboration with Mark Viney, at the time at the University of Bristol, now Universityof Liverpool, Nemetschke et al. 2010) and we established a protocol for chemical mutagenesis of S. ratti (Guo et al. 2015). We devised a protocol for RNAi gene knock down in S. ratti (Dulovic and Streit 2019). We were part of the Strongyloides genome consortium (Hunt et al. 2016) and we followed up the genomic work with transcriptomic (mRNA and small RNA) studies (Baskaran, Jaleta et al. 2017; Jaleta et al. 2017a; Holz et al. 2017) and we optimised the methods for single worm genotyping and whole genome sequencing (Zhou et al. 2019b).
Life cycle switches
Strongyloides spp. have intersting life history switches. First, a parthenogentic female (the parasitic female) produces progeny of two sexes (male and female) that differ in their chromosomal make up. Second, female progeny of the parasitic females swich between developing into infective third stage larvae (iL3s) and, upon entry into a host, into adult parasites and becoming free-living sexually reproducing adults. Interstingly the progeny of the free-living generation do not have these options. They are invariably female and develop into iL3s.
Sex determination and sex chromosomes
The sex determining mechanisms vary within the genus Strongyloides. There are species with true sex chromosomes such that individuals with two X chromosomes (plus two pairs of autosomes) are female and individuals with one X are male. Other species, for example S. papillosus have only two pairs of chromosomes, one of which is considerably larger than the other. Already more tan 40 years ago it was speculated that this is the result of a fusion of the X chromosome with one of the autosomes. In old, cytological studies some authors found no chromosomal differences between the sexes of S. papillosus. Others described that in males a portion of one chromosome is eliminated, thereby creating a hemizygous region (sex specific chromatin diminution). By combining cytological and molecular genetic approaches, we demonstrated that in S. papillosus males an internal portion of one of the two larger chromosomes is eliminated. Further we showed that the region undergoing chromatin diminution contains a high number of genes and is homologous to the X chromosome of S. ratti. The portions of the longer chromosome that is not diminished corresponds to chromosome number I of S. ratti (Nemetschke et al. 2010). These findings, in combination with comparative studies we performed in collaboration with Warwick Grant (La Trobe University) on the sister taxon Parastrongyloides trichosuri (Kulkarni et al. 2013), as well as the whole genome sequences of multiple species of Strongyloides (Hunt et al. 2016) strongly support the chromosome fusion hypothesis .
We attempt to understand, how it is achieved that all larvae produced by the free-living generation are female. So fare we showed that in S. papillosus genetically male determining mature sperm is not formed (Nemetschke, 2010; Kulkarni, 2016a). Surprisingly, in S. ratti null-X sperm and also some very early embryos with a male karyotype appear do exist but the male embryos may be unviable (Kulkarni, 2016a). In order to further investigate how the production of male determinign sperm is avoided, we conducted a detaild analysis of the spermatgenesis im S. papillosus and S. ratti and for comparison, in Parastrongyloides trichosuri, which does produce male progeny (Dulovic et al. 2022).
The parasitic - free-living switch
This switch is considered to be homologous to the switch between dauer and non-dauer development in some free-living nematodes like C. elegans or Pristionchus pacificus. Using pharmacological and RNAi experiments we could show that, like in C. elegans and P. pacificus, the nuclear hormone receptor DAF-12 is a key player in this switch in Strongyloides spp. in the progeny of the parasitic and of the free-living generations (Ogawa et al. 2009; Dulovic and Streit 2019).
People involved in this project:
Present:
- Veroni de Ree
- Dorothee Harbecke
- Xiaoxiao Yin
Past:
- Alex Dulovic
- Anna Dyka
- Alexander Eberhardt
- Li Guo
- Anja Holz
- Tegegn Jaleta
- Arpita Kulkarni
- Linda Nemetschke
- Siyu Zhou
- Olga Zhukova
Strongyloides stercoralis in humans and animals, in particular dogs - is Strongyloidiasis a zoonotic disease
Strongyloidiasis, a human disease caused by mainly Strongyloides stercoralis, is a neglected tropical disease. It is however, not limited to tropical regions. It was long known that at least some human derived S. stercoralis are capable of infecting dogs. However, it is unclear if dogs, and may be other animals, play an important role as a source for S. stercoralis infecting humans (for review see Bradbury and Streit 2024). In collaboration with more applied parasitologists we compare S. stercoralis isolated from humans and animals, in particular dogs, using molecular genetic and genomic approaches (Schär, Guo et al. 2014, Jaleta, Zhou et al. 2017, Zhou et al. 2019a, Aupalee et al. 2020a, Beiromvand et al. 2024, de Ree et al. 2024). We found that, in the wild, dogs carry human type S. stercoralis, in addition to at least one presumably dog specific species or subspecies. Also among S. stercoralis populations in humans we found biological and genomic differences that rise the question if really all S. stercoralis (s.l) in humans are the same species.
As a basis for the further investigation of the differences between poulations observed in the field, we started to establish a collection of S. stercoralis wild isolates. In addition to collecting ourselves, we hope to encourage people encountering S. stercoralis to contribute their isolates. Isolates are cultured in gerbils and frozen alive. For each isolate we will determine a set of parameters (e.g. life cycle preference, sex ratio) and the whole genome sequence. Selected isolates will be crossed in order to evaluate their species status. The isolates will be made available to the community.
People involved in this project:
Present:
- Veroni de Ree
- Dorothee Harbecke
- Sandra Gyarteng
- Xiaoxiao Yin
Past:
- Li Guo
- Tegegn Jaleta
- Siyu Zhou
- Kittipatt Aupalee
Population biology of filarial nematodes of the genus Onchocerca
The filarial nematode Onchocerca volvulus causes human onchocerciasis, commonly known as river blindness. Most species of Onchocerca, however, are parasites of ungulates. One of them, O. ochengi, is a parasite of cattle that has been estimated to be evolutionarily separated from O. volvulus by as little as 10 000 years. Like O. volvulus, O. ochengi females induce nodules in the skin of the host and the two species share the black fly Simulium damnosusm as vector. O. ochengi in cattle is therefore an attractive animal model to study various aspects of the biology of O. volvulus. Zoonotic cases in humans with various, normally animal parasitic, Onchocerca spp. do occur sparadically. We participate, mainly as the molecular arm, in projects to investigate the population biology and taxonomy of Onchocerca sp. in close collaboration with the group of Alfons Renz from the University of Tübingen and the Programme Onchocercoses in Ngoundéré, Cameroon (see (Hildebrandt et al. 2012/2014; Eisenbarth et al. 2014; Jaleta et al. 2018) and with the group of Atiporn Saeung from the Chiang Mai University in Thailand (see Aupalee et al. 2020b; Hunang et al. (2021).
People involved in this project:
Present:
Past:
- Julia Hildebrandt
- Tegegn Jaleta
- Kittipatt Aupalee
Previous Projects
- Spatiotemporal control of the Hox gene ceh-13 in the nematode
- If females can reproduce by themselves, why are there males?
Publications
Piskobulu, V., Athanasouli, M., Witte, H., Feldhaus, C., Streit, A. and Sommer, R, (2025). High nutritional conditions influence feeding plasticity in Pristionchus pacificus and render worms non-predatory. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution, 344, 94-111.
de Ree, V., Nath, T. C., Harbecke, D., Barua, P., Lee, D., Rödelsperger, C. and Streit, A. (2024). Genomic analysis of Strongyloides stercoralis and S. fuelleborni in Bangladesh. PLOS Neglected Tropical Diseases, 8(9): e0012440. https://doi.org/10.1371/journal.pntd.0012440.
Beiromvand, M., Ashiri, A., de Ree, V., Harbecke, D., Rödelsperger, C., Streit, A.* and Rafiei, A. (2024). Strongyloides stercoralis Genotyping in Human Population of Southwestern Iran. Parasites and Vectors 17:21, https://doi.org/10.1186/s13071-023-06103-6 *Corresponding author
Bradbury, R. S. and Streit, A. (2024). Is strongyloidiasis a zoonosis from dogs? Philosophical Transactions of the Royal Society B 379:20220445, doi:10.1098/rstb.2022.0445 (special issue on Strongyloides: -omics to worm free populations).
Al-Jawabreh, R., Anderson, R., Atkinson, L.E., Bickford-Smith, J., Bradbury, R.S., Breloer, M., Bryant, A.S., Buonfrate, D., Cadd, L.C., Crooks, B., Deiana, M., Grant, W., Hallem, E., Hedtke, S.M., Hunt, V., Khieu, V., Kikuchi, T., Kounosu, A., Lastik, D., van Lieshout, L., Liu, Y., McSorley, H.J., McVeigh, P., Mousley, A., Murcott, B., Nevin, W.D., Noskova, E., Pomari, E., Reynolds, K., Ross, K., Streit, A., Suleiman, M., Tiberti, N. and Viney, M. (2024). Strongyloides Questions - a Research Agenda for the Future. Philosophical Transactions of the Royal Society B, 379:20230004, doi:10.1098/rstb.2023.0004 (special issue on Strongyloides: -omics to worm free populations).
Buonfrate, D., Hunt, V. L., Odermatt, P. and Streit, A. (2024). Strongloides: omics to worm-free populations. Philosophical Transactions of the Royal Society B 379:20220448, doi:10.1098/rstb.2022.0448 (editorial for special issue on Strongyloides: -omics to worm free populations).
Paguem, A., Kamtsap, P., Manchang T. K., Yembo, J., Achukwi, M. D., Streit, A. and Renz, A. (2023). Species identity and phylogeny of Paramphistomoidea Fischoeder, 1901 occurring in cattle and sheep in North Cameroon. Veterinary Parasitology: Regional Studies and Reports 45:100922, doi:10.1016/j.vprsr.2023.100922.
Dulovic, A., Koch, I., Hipp, K. and Streit A. (2022). Strongyloides spp. eliminate male-determining sperm post-meiotically. Molecular and Biochemical Parasitiology, 251:111509 (special issue on Strongyloides research in the post genomics era).
Streit, A. (2022). What do rescue experiments with heterologous proteins tell us and what not? Parasitology Research 121:1131-1135 (Opinion, special issue on "New technologies in parasitology"). Full Text PDF
Dulovic, A., Norman, M., Harbecke, D. and Streit, A. (2022). Chemotactic and temperature-dependent responses of the Strongyloidoidea superfamily of nematodes. Parasitology,149, 1126-123.
Huang, F., Srisuka, W., Aupalee, K., Streit, A., Fukuda, M., Pitasawat, B., Junkum, A., Saingamsook, J., Somboon, P.,Takaoka, H. and Saeung, A. (2021). Diversity of nematodes infecting the human-biting black flies, Simulium nigrogilvum (Diptera: Simuliidae) in central Thailand. Acta Tropica, 224:106140.
Yalcindag, E., Stuart, P., Hasegawa, H., Streit, A., Doležalová, J., Morrogh-Bernard, H., Cheyne, S. M., Nurcahyo, W. and Foitová, I. (2021). Genetic Characterization of Nodular Worm Infections in Asian Apes. Scientific Reports, 11:7226. https://doi.org/10.1038/s41598-021-86518-2
Streit, A. (2021). Strongyloidiasis - Really a Zoonosis? In: Mehlhorn, H. and Strube, C. (Eds), Dog parasites endangering human health. Springer, Cham. pp. 195-226. https://doi.org/10.1007/978-3-030-53230-7
Dulovic, A., Renahan, T., Röseler, W., Rödelsperger, C, and Streit, A. (2020). Rhabditophanes diutinus a Parthenogenetic Clade IV Nematode with Dauer Larvae. PLOS Pathogens, 16(12): e1009113. https://doi.org/10.1371/journal.ppat.1009113.
Aupalee, K., Saeung, A., Srisuka, W., Fukuda, M., Streit, A. and Takaoka, H. (2020b). Seasonal Filarial Infections and Their Black Fly Vectors in Chiang Mai Province, Northern Thailand. Pathogens, 9:521. https://doi.org/10.3390/pathogens9060512.
Paguem, A., Abanda B., Ngwasiri, N. N., Eisenbarth, A., Renz, A., Streit, A. and Achukwi, M. D. (2020). Host Specificity and Phylogeny of Trichostrongylidae of Domestic Ruminants in the Guinea savannah of the Adamawa Plateau in Cameroon. Veterinary Parasitology: Regional Studies and reports, 21:100412.
Aupalee, K., Wijit, A., Singphai, K., Roedelsperger, C., Zhou, S., Saeung, A. and Streit, A. (2020a). Genomic studies on Strongyloides stercoralis in northern and western Thailand. Parasites & Vectors, 13:250. https://doi.org/10.1186/s13071-020-04115-0.
Zhou, S., Harbecke, D. and Streit, A. (2019b). From the feces to the genome: a guideline for the isolation and preservation of Strongyloides stercoralis in the field for genetic and genomic analysis of individual worms. Parasites & Vectors, 12:496. https://doi.org/10.1186/s13071-019-3748-5.
Zhou, S., Fu, X., Pei, P., Kucka, M., Liu, J., Tang, L., Zhan, T., He, S., Chan, Y., Rödelsperger, C., Liu, D. and Streit, A. (2019a). Characterization of a non-sexual population of Strongyloides stercoralis with hybrid 18S rDNA haplotypes in Guangxi, Southern China. PLOS Neglected Tropical Diseases,13(5): e0007396. https://doi.org/10.1371/journal.pntd.0007396.
Dulovic, A. and Streit, A. (2019). RNAi-mediated knockdown of daf-12 in the model parasitic nematode Strongyloides ratti. PLOS Pathogens, 15(3): e1007705. https://doi.org/10.1371/journal.ppat.1007705. Step by step protocol.
Jaleta, T. G., Roedelsperger, C., Abanda, B. Eisenbarth, A., Achukwi, M. D, Renz, A. and Streit, A. (2018). Full mitochondrial and nuclear genome comparison confirms that Onchocerca sp. "Siisa" is Onchocerca ochengi. Parasitology Research, 117, 1069-1077.
Holz, A. and Streit, A. (2017). Gain and loss of small RNA classes - characterization of small RNAs in the parasitic nematode family Strongyloididae. Genome Biology and Evolution, 9, 2826-2843. https://doi.org/10.1093/gbe/evx197
Jaleta, T. G., Zhou, S., Bemm, F., Khieu, V., Sinuon, M., Schär, F., Odermatt, P. and Streit, A. (2017b). Different but overlapping populations ofStrongyloides stercoralis in dogs and humans - dogs as a possible source for zoonotic strongyloidiasis. PLOS Neglected Tropical Diseases, 11(8): e0005752. https://doi.org/10.1371/journal.pntd.0005752
Jaleta, T., G., Roedelsperger, C. and Streit, A. (2017a). Parasitological and transcriptomic comparison of Strongyloides ratti infections in natural and in suboptimal permissive hosts. Experimental Parasitology, 180, 112-118 (special issue "Proceedings of the 27th Meeting of the German Society for Parasitology 2016"). DOI:10.1016/j.exppara.2016.12.003
Streit, A. (2017). Genetics: Modes of Reproduction and Genetic Analysis. Parasitology, 144, 316-326 (special issue on Strongyloides spp.). DOI:10.1017/S0031182016000342
Baskaran, P., Jaleta, T. G., Streit, A. and Rödelsperger, C. (2017). Duplications and positive selection drive the evolution of parasitism-associated gene families in the nematode Strongyloides papillosus. Genome Biology and Evolution, 9, 790-801.
Streit, A. and Davis, R. E. (2016). Chromatin diminution version 2. In: eLS. John Wiley & Sons Ltd, Chichester. http://www.els.net/, DOI: 10.1002/9780470015902.a0001181.pub2 (Review).
Kulkarni, A., Lightfoot, J. W. and Streit, A. (2016b). Germline organization in Strongyloides nematodes reveals alternative differentiation and regulation mechanisms. Chromosoma 125, 725-745. doi:10.1007/s00412-015-0562-5
Dulovic, A., Puller, V. and Streit, A. (2016) Optimizing culture conditions for free-living stages of the nematode parasite Strongyloides ratti. Experimental Parasitology 168, 25-30. 10.1016/j.exppara.2016.06.005.
Streit, A., Wang, J., Kang, Y. and Davis, R. E. (2016). Gene Silencing and Sex Determination by Programmed DNA Elimination in Parasitic Nematodes. Current opinion in Microbiology 32 120-127 (Review).
Hunt, V. L., Tsai, I. J., Coghlan, A., Reid, A. J., Holroyd, N., Foth, B. J., Tracey, A., Cotton, J. A., Stanley, E. J., Beasley, H., Bennett, H., Brooks, K., Harsha, B., Kajitani, R., Kulkarni, A., Harbecke, D., Nagayasu, E., Nichol, S., Ogura, Y., Quail, M., Randle, N., Ribeiro, D., Sanchez-Flores, A., Hayashi, T., Itoh, T., Denver, D. R., Grant, W., Stoltzfus, J. D., Lok, J. B., Murayama, H., Wastling, J., Streit, A., Kikuchi, T., Viney, M. E., Matthew Berriman, M. (2016). The Genomic Basis of Parasitism in the Strongyloides Clade of Nematodes. Nature Genetics 48, 299-307. doi:10.1038/ng.3495
Kulkarni, A., Holz, A., Rödelsperger, C., Harbecke, D. and Streit, A. (2016a). Differential chromatin amplification and chromosome complements in the germline of Strongyloididae (Nematoda). Chromosoma 125, 125-136. doi:10.1007/s00412-015-0532-y
Guo, L., Chang, Z., Dieterich, C. and Streit, A. (2015). A protocol for chemical mutagenesis in Strongyloides ratti. Experimental Parasitology 158, 2-7 (special issue). .
Witte, H., Moreno, E., Rödelsperger, C., Kim, J. S., Kim, J. S., Streit, A. and Sommer, R. J. (2015). Gene inactivation using the CRISPR/Cas9 system in the nematode Pristionchus pacificus. Development Genes and Evolution 255, 55-62.
Hildebrandt, J. C., Eisenbarth, A., Renz, A. and Streit, A. (2014). Reproductive biology of Onchocerca ochengi, a nodule forming filarial nematode in zebu cattle. Veterinary Parasitology 205, 318-329.
Streit, A. (2014). How to become a parasite without sex chromosomes: a hypothesis for the evolution of Strogyloides sp. and related nematodes. Parasitology 141, 1244-1254 (Review).
Schär, F., Guo. L., Streit, A., Khieu, V., Sinuon, M., Marti, H and Odermatt, P. (2014). Strongyloides stercoralis genotypes in humans in Cambodia. Parasitology Inernational 63, 533-536.
Kulkarni, A., Dyka, A., Nemetschke, L., Grant, W. N. and Streit, A. (2013). Parastrongyloides trichosuri suggests that XX/XO sex determination is ancestral in Strongyloididae (Nematode). Parasitology 140, 1822-1830.
Rödelsperger, C. and Streit, A. (2013). Komplexität im Kleinen - Nematoden-Genome im Vergleich. BIOspektrum 6/13, 606-610 (in German).
Eisenbarth, A., Ekale, D., Hildebrandt, J. C., Achukwi, M. D, Streit, A. and Renz, A. (2013). Molecular evidence of ‘Siisa form’, a new genotype related to Onchocerca ochengi in cattle from North Cameroon. Acta Tropica 127, 261-265.
Rödelsperger, C., Streit, A. and Sommer, R. J. (2012). Structure, function and evolution of the nematode genome. In: eLS. John Wiley & Sons Ltd, Chichester. http://www.els.net/ DOI: 10.1002/9780470015902.a0024603 (Review).
Streit, A. (2012) Silencing by throwing away: a role for chromatin diminution. Developmental Cell 23, 918-919 (Preview).
Hildebrandt, J. C., Eisenbarth, A., Renz, A. and Streit, A. (2012). Single worm genotyping demonstrates that Onchocerca ochengi females simultaneously produce progeny sired by different males. Parasitology Research 111, 2217-2221.
Sommer R. J. and Streit, A. (2011). Comparative Genetics and Genomics of Nematodes: Genome Structure, Development and Lifestyle. Annual Review of Genetics 45, 1-20 (Review).
Nemetschke, L., Eberhardt, A. G., Hertzberg, H. and Streit, A. (2010). Genetics, chromatin diminution and sex chromosome evolution in the parasitic nematode genus Strongyloides. Current Biology 20, 1687-1696.
Streit, A. and Sommer, R. J. (2010). Genetics: Random expression goes binary. Nature 463, 891-892 (News and Views).
Nemetschke, L., Eberhardt, A. G., Viney, M. E. and Streit, A. (2010). A genetic map of the animal-parasitic nematode Strongyloides ratti. Molecular and Biochemical Parasitiology 169, 124-127.
Wegewitz, V., Schulenburg, H. and Streit, A. (2009). Do males facilitate the spread of novel phenotypes within populations of the androdioecious nematode Caenorhabditis elegans? Journal of Nematology 41, 247-254.
Minasaki R., Puoti A. and Streit A. (2009). The DEAD-box protein MEL-46 is required in the germ line of the nematode Caenorhabditis elegans. BMC Developmental Biology 9, 35.
Ogawa, A., Streit, A., Antebi, A. and Sommer, R. J. (2009). A conserved endocrine mechanism controls the formation of dauer and infective larvae in nematodes. Current Biology 19, 67-71.
Summary of this article, written for a broad audience (in German). Appeared in the newsletter of the German Society for Parasitology 1/2009.
Eberhardt, A. G., Mayer, W. E., Bonfoh, B. and Streit, A. (2008). The Strongyloides (Nematoda) of sheep and the predominant Strongyloides of cattle form at least two different, genetically isolated populations. Veterinary Parasitology 157, 89-99.
Wegewitz, V., Schulenburg, H. and Streit, A. (2008) Experimental insight into the proximate causes of male persistence variation among two strains of the androdioecious Caenorhabditis elegans (Nematoda). BMC Ecology 8, 12.
Streit, A. (2008). Reproduction in Strongyloides (Nematoda): a life between sex and parthenogenesis. Parasitology 135, 285-294 (Review).
Eberhardt, A. G., Mayer, W. E. and Streit, A. (2007). The free-living generation of the nematode Strongyloides papillosus undergoes sexual reproduction. International Journal for Parasitology 37, 989-1000.
Minasaki, R. and Streit, A. (2007). mel-47, a novel protein required for early cell divisions in the nematode Caenorhabditis elegans. Molecular Genetics and Genomics 277, 315-328.